BD Announces $24M Investment to Manufacture COVID-19 Diagnosic Tests
Capital investments will enable U.S. manufacturing of 8 million BD Veritor™ brand SARS-CoV-2 tests per month
On Jul 31, 2020Franklin Lakes-based BD (Becton, Dickinson and Company), a global medical technology company, announced a $24 million investment from the U.S. Department of Defense in collaboration with the U.S. Department of Health and Human Services to support the scale up of U.S. manufacturing capabilities for BD Veritor™ Solution for Rapid Detection of SARS-CoV-2.
The additional capital equipment will bolster domestic production and increase total production capacity by 50 percent. These investments will enable global production of more than 12 million test kits per month by the end of February 2021.
“Making COVID-19 diagnostic tests widely available is critical to expanding rapid detection of COVID-19 infections, and mitigating the impact of the disease by identifying affected patients, quickly quarantining infectious individuals and tracing their contacts,” said Dave Hickey, president of Integrated Diagnostic Solutions for BD. “This investment will bolster our U.S. manufacturing capabilities helping us quickly scale our production of point-of-care COVID-19 tests to ensure we have a robust supply for our U.S. customers.”
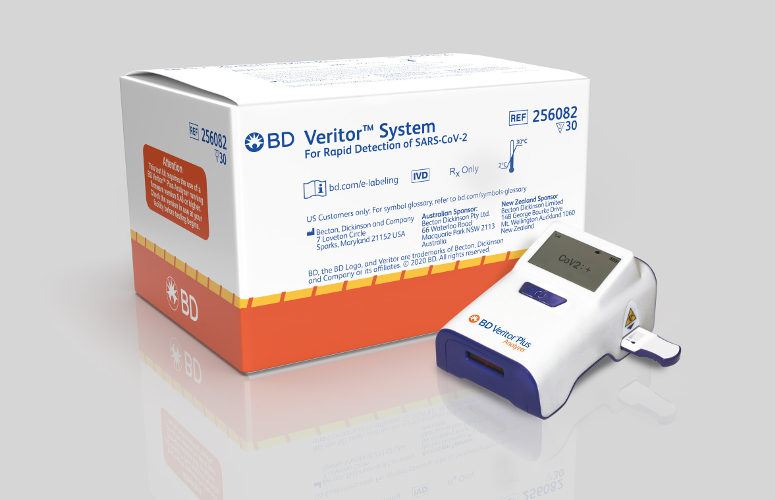
BD announced that it had received FDA emergency use authorization for the BD Veritor™ Plus SARS-CoV-2 antigen assay on July 6, 2020 and plans to leverage its growing U.S. installed base of more than 25,000 BD Veritor™ Plus instruments to enable the deployment of the SARS-CoV-2 assay across the U.S. The easy-to-use design of the instrument, slightly larger than a cell phone, makes it ideal for use in a variety of clinical settings including hospitals, clinician offices, urgent care centers, and retail pharmacies, where it has already been used in influenza, group A strep and RSV testing for several years.
To access more business news, visit NJB News Now.