
High-Tech Medical Devices Offer Solutions
New Jersey medical technology companies offer high-tech devices to improve healthcare.
By Anthony Bucci, Assistant Editor On Jun 14, 2014The healthcare industry in the United States has been going through a drastic change. With the introduction of the Affordable Care Act, more Americans have access to quality care, but some are facing rising costs. Additionally, new medical technologies and developments are changing the way healthcare is delivered, aiming to reduce escalating costs, while improving patient outcomes.
For decades, New Jersey has been known as the “Medicine Chest of the World,” and Garden State medical technology companies have been spending billions of dollars on research and development (R&D) – introducing products to aid doctors, nurses and hospitals in order to provide the highest-quality, most affordable care possible. In this article, New Jersey Business looks at a handful of medical products recently introduced by leading medical technology companies that are either based in the state, or have a large presence here.
BD
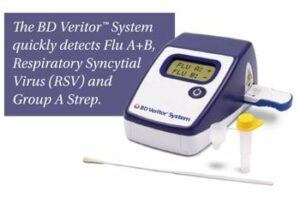
“What makes the BD Veritor so great is that when a patient doesn’t feel well or is experiencing flu-like symptoms, by taking a cotton-swabbed sample from inside of their nasal cavity, an answer as to what they have can be had in 10 minutes,” says William Kozy, BD’s executive vice president and chief operating officer. “This way, the doctor knows exactly what to do for [a patient] and quickly provide treatment.”
Kozy says that a lot of patient care is migrating outside of the hospital environment to more cost effective sites, and the Veritor System is aiding in that regard.
“More and more healthcare delivery is being done outside of the hospital, and that includes some of the diagnostics testing,” he says. “There are even ‘one minute clinics’ at pharmacies, where people are walking in with what they believe is a relatively simple affliction, but it gets taken care of right there in a brief period of time. This is an example of healthcare moving away from the hospital with a very innovative diagnostic tool to help diagnose ailments before people go home.”
Franklin Lakes is home to BD’s global headquarters and houses three of the company’s six worldwide business units: Medical Surgical Systems, Preanalytical Systems and Diabetes Care and the R&D for those units.
Abbott Point of Care

The original i-STAT system was introduced in 2011, however, Sharon Bracken, president of Abbott Point of Care, notes that the company is continuing “to expand i-STAT’s capabilities – in terms of the number of tests available, newer features such as ergonomic grips for the [machine’s] cartridge and ongoing advances in software features.”
Recently, Abbott has improved the efficiency of the i-STAT. The newer i-STAT 1 Wireless System – only available in the US – “potentially saves precious time by allowing doctors and nurses to perform critical tests and transmit test results directly from the patient’s bedside to the patient’s electronic medical records, helping doctors make important treatment decisions sooner. This rapid transfer of information can improve patient care and lead to quicker discharges if further treatment is unneeded,” Bracken says.
Overall, the i-STAT system is currently used in more than 2,000 hospitals in 84 different countries, and Bracken says it can also improve health system efficiencies and reduce unnecessary costs. “For example, by moving cardiac marker testing to the bedside with the i-STAT System, one university in Oklahoma saved 10 minutes for every patient presenting to the emergency department with chest pain,” she says. “This translated to an estimated savings of $180,000 per year.”
Abbott Point of Care has roughly 1,400 employees across the globe. In New Jersey, it has approximately 200 employees dedicated to its Point of Care business, including mechanical, electrical and software engineers, quality assurance and regulatory experts, sales and marketing groups, and technical support and customer service staff.
Novo Nordisk

One if its newest innovations launched in early 2014, is the NovoPen Echo®, a device that delivers insulin to patients with diabetes. According to the company, it is the first and only pen device available in the US with half-unit dosing and a memory function that records the dose and time passed since the last injection.
“The NovoPen Echo® fills a real need in diabetes care,” says Todd Hobbs, chief medical officer for Novo Nordisk in North America. “Although there isn’t a large amount of patients that need a half-unit dosing of insulin, for those that do need it, it is critical. Especially for children who only require a half-dose and adults who are fine-tuning their meals and need more control of their blood sugar.”
Hobbs says the memory function is an important feature because there are times that adults can become distracted when they need to take insulin and are unaware if they injected it or not.
“The device lets them see the previous dose and when they took it,” he says. “And with children, the memory function allows parents and caregivers to look back to confirm that a dose was delivered and if the dose was correct.”
Because Novo Nordisk develops drugs as well as medical devices, Hobbs mentions that generally, the process of pushing devices to market from initial R&D stages is more “timely” than that of a drug.
“There are mechanical concepts that have to go into these devices, so we have to see if the product can be mass-produced, but there are bigger issues as well,” he says. “Is the device accurate? We have to deliver an extremely accurate reproducible device. We have to do safety testing, efficiency testing. Then we have to do quite a bit of human factors testing.”
Stryker
Mahwah is home to Stryker Corporation’s orthopaedics and spine divisions as well as sales and distribution offices and other corporate campuses.
The company manufactures and sells products to treat a wide range of medical conditions affecting the musculoskeletal system, with products to treat bones and joints all over the body, including hips, knees, shoulders, elbows and ankles.
Stryker Orthopaedics is made up of several businesses that have introduced innovative products to the marketplace. A few recent examples of the technological advances from the division include the Stryker Mobile Bearing Hip™ System, a dual mobility implant that works in combination with Stryker’s X3 Precisely Engineered Polyethylene.
Stryker’s Accolade II is a Morphometric tapered hip replacement stem, that according to the company, has been designed to address a changing patient population through 556 CT scans that are part of a proprietary technology called SOMA (Stryker Orthopaedics Modeling and Analytics system) and accommodates a variety of surgical approaches.
Additionally, the Triathlon Tritanium Cementless system allows surgeons to use total knee replacement in a variety of patients. Stryker says Tritanium implants are the latest evolutions in the Triathlon Cementless knee portfolio, and are manufactured with Stryker’s 3D printing technology.
Stryker has 37 manufacturing and R&D centers around the world. In 2013, the company spent $536 million on R&D and the list of Stryker patents owned globally grew to 5,203, according to the company. The Mahwah-based Orthopaedic division’s headquarters includes R&D, global manufacturing, marketing and the Homer Stryker Center, which Stryker says, is dedicated to advancing orthopaedic education and innovation.
Conclusion
The products mentioned are only a minute fraction of new medical devices currently on the market. Furthermore, medical technology companies in New Jersey are continuously developing and introducing new products and innovative solutions to not only help ease the burden on the US healthcare system – but perhaps even more importantly – aid in the health and well-being of the people in need of these devices.
Related Articles:





