NJ Life Sciences Companies Tackle COVID-19
A review of how drug and related diagnostic tech firms are at the forefront of pandemic cure initiatives.
By Meg Fry, Contributing Writer On Jun 10, 2020New Jersey, home to 13 of the 20 largest pharmaceutical, medical technology, and diagnostics companies in the world, also has the highest concentration of scientists and engineers on the planet.
Still, the novel coronavirus disease (COVID-19) that has affected millions worldwide, drastically shut down the state for much of the spring, with New Jersey experiencing more than 137,000 cases and more than 9,100 fatalities in the first two months of the pandemic.
“There has been an unusual convergence in that our state, one of the global hubs for medical innovation, has been impacted so disproportionately by this dreaded condition,” says Dean J. Paranicas, president and CEO of the HealthCare Institute of New Jersey (HINJ), a New Bruswick-based statewide trade association for biopharmaceutical and medical technology companies.
With no known vaccine, treatment or cure, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has dominated the life sciences industry since the virus’ discovery in Wuhan, China in December.
By April, there were more than 60 companies in New Jersey working on more than 170 programs to address COVID-19, says Debbie Hart, president and CEO of BioNJ, a statewide advocate organization for the biotechnology and life sciences industries in Trenton.
“We’ve witnessed extraordinary commitments by companies to the larger effort, including retaining and supporting their employees in different ways during this difficult time, looking within their own portfolios for ways to help, donating personal protective equipment (PPE), critical medication, and other supplies, and making significant financial donations,” Hart says.
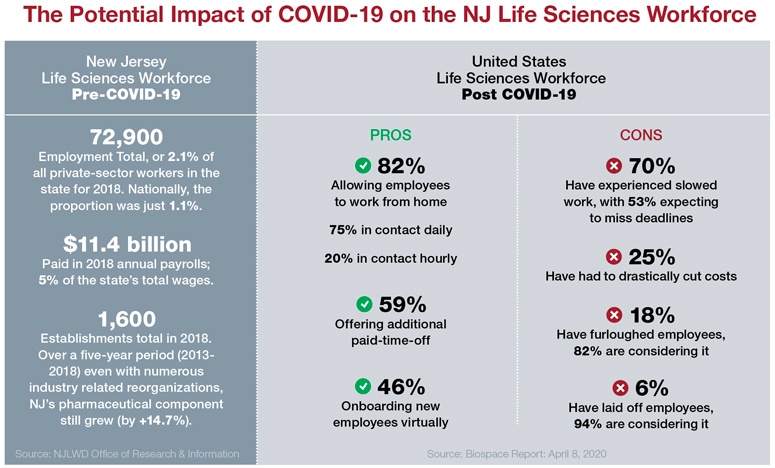
Hart says BioNJ has been working hard to connect companies with similar resources.
“The flood gates have opened in terms of the requests for assistance we’ve received – and we’re happy to respond,” she says.
If there is a positive in the battle against COVID-19, the immediate increased access to – and more effective communication with – global health authorities would be it, as it has accelerated regulatory reviews and approvals.
It also encouraged the creation of new collaborations while strengthening and expanding existing partnerships.
Paranicas says he hopes this may pave easier paths for other life-changing medical discoveries in the future.
“It further reinforces the critical element of medical innovation as a primary means to respond,” he says.
Read on to discover what New Jersey companies have been doing since COVID-19 established a “new normal” in the state.
Diagnostic Testing
In March, the Hackensack Meridian Health Center for Discovery and Innovation in Nutley launched a rapid response coronavirus test, reducing the testing process from days to hours.
By April, BD had developed three tests to rapidly detect COVID-19, a point-of-care test with BioMedomics to confirm antibodies in as little as 15 minutes, and a handheld, portable device to help increase testing capabilities.
“What typically takes two to three years has been done in a matter of weeks,” says Troy Kirkpatrick, senior director of public relations at Becton, Dickinson and Co. (BD), a 123-year old, multibillion-dollar, global medical device company in Franklin Lakes.
BD also continues to increase infection surveillance technology in hospitals to help warn of future COVID-19 spikes, Kirkpatrick says.
Also in April, Rutgers University Cell and DNA Repository (RUCDR) Infinite Biologics in Piscataway, Spectrum Solutions (a provider and manufacturer of medical and clinical biosample collection devices and collection kits), and Accurate Diagnostic Labs (a medical laboratory), developed a saliva collection kit for COVID-19, which reduces healthcare workers’ exposure to those being testing for COVID-19. Last Month, the test kit received an amended emergency use authorization from the FDA.
Vaccine Candidates
Numerous companies with a New Jersey presence continue to develop COVID-19 vaccine candidates, including OncoSec Medical, a biotechnology company in Pennington; Sanofi, a global biopharmaceutical company with US headquarters in Bridgewater; and Pfizer, a global biopharmaceutical company based in New York City.
But it was Johnson & Johnson (J&J), the 134-year old, multibillion-dollar global healthcare company in New Brunswick, that made headlines when subsidiary Janssen Pharmaceuticals expanded its partnership with the Biomedical Advanced Research and Development Authority (BARDA) to commit more than $1 billion toward COVID-19 research and development. In late March, J&J announced the selection of a lead COVID-19 vaccine candidate from constructs it has been working on since January 2020. The company expects to initiate human clinical studies at the latest by September 2020 and anticipates the first batches of a COVID-19 vaccine could be available for emergency use authorization in early 2021.
“In January, when the genetic sequence of the novel coronavirus was made available, J&J immediately began working on a vaccine, leveraging the platform that we’ve used against other diseases,” says Dr. Richard Nettles, vice president of US medical affairs at Janssen Infectious Diseases.
Janssen’s proven AdVac technologies previously have rapidly developed and manufactured new vaccine candidates, including against Ebola, Zika, RSV and HIV.
Smaller companies, such as Soligenix Inc., a biopharmaceutical company that employs nearly 15 people in Princeton, are also making progress with existing vaccine platforms.
“We believe creating a vaccine with enhanced stability at elevated temperatures that can obviate the costs and logistical burdens associated with cold chain storage and distribution has the potential to provide a distinct advantage over other vaccines currently in development and simplifies worldwide distribution,” Christopher Schaber, president and CEO of Soligenix, says.
Soligenix’s ongoing collaborations to assess potential coronavirus and filovirus vaccines, including with the University of Hawaii and the University of Colorado, have significantly progressed.
“Vaccines based on protein sub-units, like CiVax, are very safe,” Schaber says. “They aren’t going to modify host DNA, they aren’t capable of causing infection under any scenario, and they’re very stable.”
Existing Therapeutics
Ongoing work has continued to screen compound libraries for molecules with antiviral activity against COVID-19.
For example, Sanofi believes its rheumatoid arthritis treatment, Kevzara, may help inhibit the inflammatory response that often accompanies COVID-19, while Novartis, a global healthcare and pharmaceuticals company, believes the same of Jakavi, an oral inhibitor used to treat conditions such as rare blood and bone marrow cancers.
Eagle Pharmaceuticals, a $48-million pharmaceuticals company in Woodcliff Lake that employs nearly 100, says it planned to commence Phase 2 clinical trials in May in partnership with Hackensack University Medical Center for RYANODEX, first approved in 2014 for malignant hyperthermia.
“Our years of research have resulted in a deep knowledge of how RYANODEX works and insights into the potential of RYANODEX to address unmet needs for multiple diseases in which calcium dysregulation plays a significant role,” Scott Tarriff, CEO of Eagle Pharmaceuticals, says. “We believe the antiviral effect observed in the laboratory suggests RYANODEX may be useful in combatting the COVID-19 public health crisis our world now faces.”
Innovative Treatments
New Jersey hospitals such as Hackensack Meridian Health in Edison and universities such as Rutgers in New Brunswick continue to advance research and conduct convalescent plasma treatments using antibodies from recovered COVID-19 patients.
But other blood-related medical technologies may also help treat the deadly inflammation often associated with COVID-19.
For example, CytoSorbents Corp., a $25-million biotechnology company in Monmouth Junction that employs more than 100, was awarded emergency use authorization for its blood purification technology, CytoSorb, on COVID-19 patients.
And BioAegis Therapeutics, a young biotechnology company in North Brunswick that employs 11, requested an accelerated clinical trial for the recombinant human plasma gelsolin therapy the company has been studying in pneumonia patients.
“Hiding in plain sight is evolution’s approach to address medical issues that have previously been seen as out of reach, that is, to prevent the damage caused by excessive inflammation without suppressing the immune system or putting patients at risk for infection,” Susan Levinson, co-founder and CEO of BioAegis Therapeutics, says.
A key regulator of the immune response to infection, those with low gelsolin levels may develop serious complications, like those often seen in COVID-19 patients.
“We are simply capitalizing on evolutionary wisdom with a natural protein, plasma gelsolin, which has fulfilled this role for vertebrates for millennia,” Levinson says.
Looking Forward
As companies adapt and develop new products for COVID-19, research for next generation products continue to help create cures for other serious conditions, despite unprecedented challenges with clinical trials and unexpected delays.
“Perhaps the next time a situation occurs like this, patient populations now at risk because of existing health conditions may be less so because of advanced medical innovations,” Paranicas says.
There is hope, Paranicas adds, that the long-term implications of this pandemic include lasting changes to how medical innovations are pursued, both from a research and development perspective, as well as with regulatory authorities.
“If you look back over history, in moments of crisis, there is often a critical reassessment of the way things have been done, how things need to be done, and how they could be done moving forward,” he says. “When you view this crisis in that kind of continuum, we may very well see how much of the current revisions and exceptions being made to existing practices will continue to evolve and adapt.”
To access more business news, visit NJB News Now.
Related Articles: